Specific Heat Capacity
Specific Heat Capacity: Overview
This topic covers concepts, such as Heat Capacity of Solids, Definition of a Calorie, Specific Heat Capacity of Water, Variation of Specific Heat Capacity of Water with Temperature, Molar Specific Heat Capacities in Terms of Gas Constant, etc.
Important Questions on Specific Heat Capacity
At a given temperature, the specific heat of a gas at constant pressure is always greater than its specific heat at constant volume.
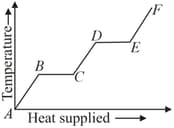
A source of heat supplies heat at a constant rate to a solid cube. The slope of portion of the graphs gives :-
Calorie is defined as the amount of heat required to raise temperature of of water by and it is defined under which of the following conditions :-
The quantities of heat required to raise the temperatures of two copper spheres of radii through 1 K are in the ratio of
At a given temperature, the specific heat of a gas at constant pressure is always greater than its specific heat at constant volume.
For three gases (assume ideal); oxygen, nitrogen and carbon dioxide, (Cp - Cv) will be
One mole of an ideal monoatomic gas at temperature T0 expands slowly according to the law If the final temperature is 2T0, heat supplied to the gas is
A copper block of mass is heated in furnace to a temperature of and then placed on a large ice block. What is the maximum amount of ice that can melt ? (Specific heat of copper is ; heat of fusion of water is )
If one mole of a monoatomic gas is mixed with one mole of a diatomic gas , the value of γ for the mixture is
of heat is required to raise the temperature of of an ideal diatomic gas at constant pressure from to . The amount of heat required (in calorie) to raise the temperature of the same gas through the same range at constant volume is
